Electronegativity Trend Explained: Periodic Behavior, Causes, and Real-World Significance
Electronegativity trend is one of the most important ideas in chemistry because it explains how atoms attract electrons and why elements behave differently in chemical reactions. From predicting bond polarity to understanding reactivity patterns across the periodic table, this concept forms the backbone of modern chemical reasoning.
Here in this post, We will explore what electronegativity really means, how it changes across periods and groups, why these changes occur, and how the trend is applied in real-world chemistry. This article is designed to be both academically solid and easy to understand, whether you’re a student, teacher, or chemistry enthusiast.
What Is Electronegativity?
Electronegativity is the ability of an atom to attract shared electrons toward itself when it forms a chemical bond. It does not measure charge or size directly; instead, it reflects how strongly a nucleus pulls on bonding electrons.
Atoms with higher electronegativity values attract electrons more strongly, while atoms with lower values tend to lose electrons more easily.
Although electronegativity is not a directly measurable physical quantity, scientists have developed several scales to estimate it, the most famous being:
- Pauling scale (most commonly used)
- Mulliken scale
- Allred–Rochow scale
On the Pauling scale:
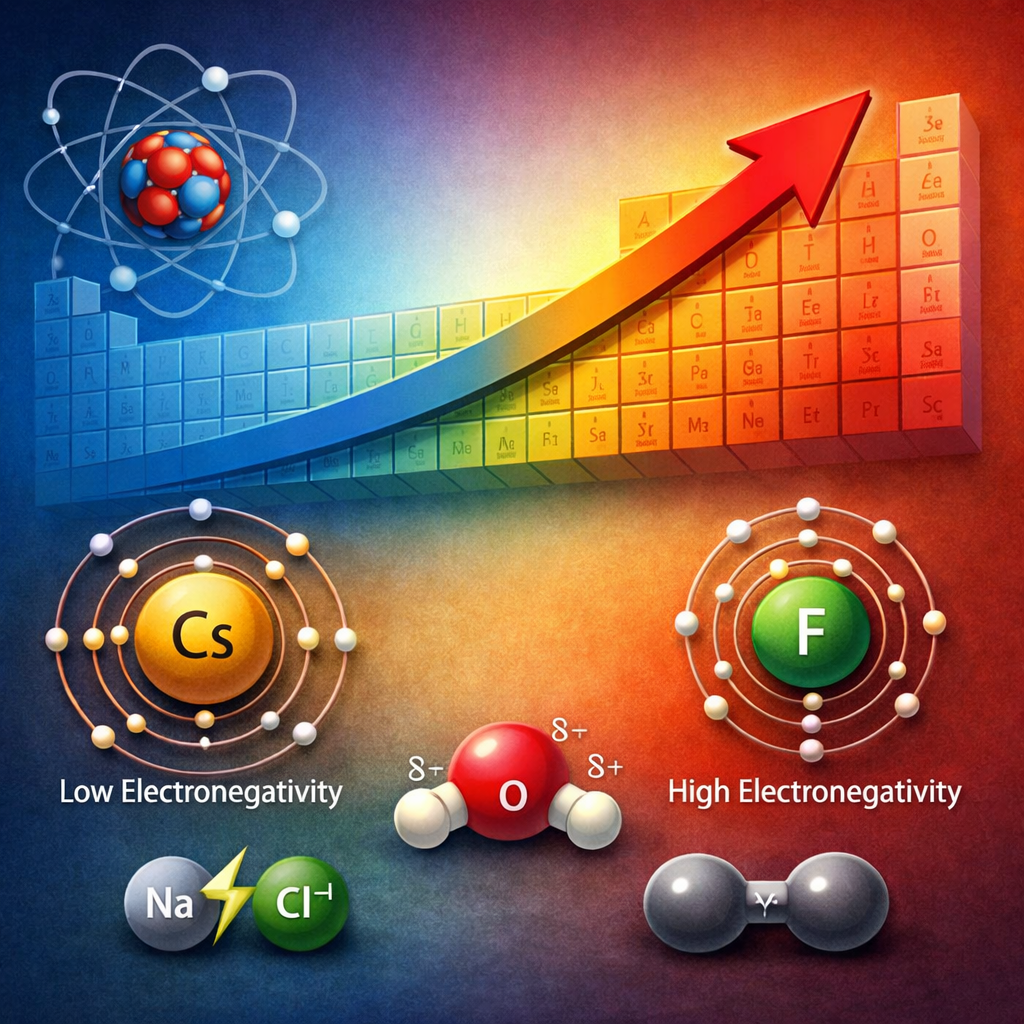
- Fluorine has the highest electronegativity (4.0)
- Cesium and francium have some of the lowest values
Understanding this property helps explain why some bonds are ionic, others covalent, and some polar in nature.
Electronegativity Trend in the Periodic Table
The electronegativity trend follows a predictable pattern across the periodic table, governed by atomic structure and nuclear forces. Once you understand this pattern, chemical behavior becomes far easier to predict.
Across a Period (Left to Right)
Electronegativity increases from left to right across a period.
Why does this happen?
- The number of protons increases
- Nuclear charge becomes stronger
- Atomic radius decreases
- Electrons are pulled closer to the nucleus
As a result, atoms on the right side of the periodic table (especially nonmetals) have a stronger attraction for electrons.
Example (Period 2):
- Lithium < Carbon < Nitrogen < Oxygen < Fluorine
Fluorine sits at the top right and exhibits the strongest pull on electrons.
Down a Group (Top to Bottom)
Electronegativity decreases as you move down a group.
Reasons include:
- Increase in atomic size
- More electron shells
- Greater shielding effect
- Reduced nuclear pull on outer electrons
Even though the nucleus has more protons, the increased distance weakens its attraction for bonding electrons.
Example (Group 17 – Halogens):
- Fluorine > Chlorine > Bromine > Iodine
What Is the Trend for Electronegativity and Why Does It Matter?
If you’re asking what is the trend for electronegativity, the simple answer is:
- It increases across a period
- It decreases down a group
But the importance goes far beyond memorization. This trend explains:
- Bond polarity
- Type of chemical bonding
- Molecular shape
- Reactivity of elements
- Strength of acids and bases
Electronegativity acts as a bridge between atomic structure and chemical behavior, making it one of the most powerful predictive tools in chemistry.
What Is the Trend of Electronegativity in Metals vs Nonmetals?
The trend of electronegativity also highlights the stark contrast between metals and nonmetals.
Metals
- Low electronegativity
- Tend to lose electrons
- Form cations
- Found on the left and center of the periodic table
Nonmetals
- High electronegativity
- Tend to gain electrons
- Form anions or share electrons
- Found on the right side of the table
Metalloids
- Intermediate electronegativity
- Show mixed behavior
- Useful in semiconductors
This distinction explains why metals and nonmetals readily form ionic compounds.
What Is the Periodic Trend for Electronegativity?
To fully answer what is the periodic trend for electronegativity, you must consider three core atomic factors:
1. Atomic Radius
Smaller atoms hold electrons closer, increasing attraction.
2. Nuclear Charge
More protons mean a stronger pull on electrons.
3. Shielding Effect
Inner electrons reduce the nucleus’s pull on outer electrons.
The periodic trend emerges from the balance between these forces. Across periods, nuclear charge dominates; down groups, shielding and size dominate.
Why Noble Gases Usually Have No Electronegativity Values
Most noble gases do not have assigned electronegativity values because they rarely form chemical bonds. Since electronegativity describes attraction in bonding situations, it becomes meaningless for inert elements.
However, heavier noble gases like xenon can form compounds, and in those rare cases, electronegativity can be estimated.
Electronegativity Trend and Chemical Bonding
The difference in electronegativity between two atoms determines the type of bond formed.
Ionic Bonds
- Large electronegativity difference
- Electron transfer occurs
- Example: Sodium chloride (NaCl)
Polar Covalent Bonds
- Moderate electronegativity difference
- Unequal electron sharing
- Example: Water (H₂O)
Nonpolar Covalent Bonds
- Little or no electronegativity difference
- Equal sharing of electrons
- Example: Oxygen gas (O₂)
This makes the electronegativity trend essential for predicting bond character.
How Electronegativity Affects Molecular Polarity
Molecular polarity depends on:
- Bond polarity (electronegativity difference)
- Molecular geometry
Even if individual bonds are polar, the molecule may be nonpolar if the dipoles cancel out (e.g., CO₂).
Conversely, molecules like water remain polar due to their bent shape and uneven charge distribution.
Relationship Between Electronegativity and Reactivity
The electronegativity trend helps explain why certain elements are highly reactive.
Highly Electronegative Elements
- Strongly attract electrons
- Highly reactive nonmetals
- Example: Fluorine
Low Electronegative Elements
- Easily lose electrons
- Highly reactive metals
- Example: Alkali metals
This explains why alkali metals react violently with halogens.
Electronegativity Trend in Acid–Base Strength
Electronegativity also influences acid and base strength:
- More electronegative atoms stabilize negative charge
- Stronger acids often have highly electronegative atoms bonded to hydrogen
Example:
- HF is weaker than HCl despite fluorine being more electronegative, due to bond strength
- Across a period, acidity increases with electronegativity
Understanding these nuances prevents oversimplification.
Common Misconceptions About Electronegativity
- Higher electronegativity does not mean larger atomic size
It’s usually the opposite. - Electronegativity is not the same as electron affinity
Electron affinity measures energy change, not attraction in bonds. - Electronegativity is context-dependent
Values can vary slightly depending on bonding environment.
Real-World Applications of the Electronegativity Trend
The impact of the electronegativity trend extends beyond textbooks:
- Material science: Predicting semiconductor behavior
- Pharmaceuticals: Designing drug molecules
- Environmental chemistry: Understanding pollutant interactions
- Biochemistry: Explaining hydrogen bonding in DNA and proteins
Hydrogen bonding, critical to life, occurs because of large electronegativity differences involving oxygen and nitrogen.
How to Remember the Electronegativity Trend Easily
Here are some memory tips:
- “Up and to the right” → higher electronegativity
- Fluorine is always the highest
- Metals lose, nonmetals gain
- Larger atoms = lower electronegativity
Visualizing the periodic table as a map of attraction forces can make the concept intuitive.
Summary
The electronegativity trend provides a unifying explanation for countless chemical phenomena. By understanding how and why electronegativity changes across the periodic table, you gain the ability to:
- Predict bond types
- Understand molecular polarity
- Explain reactivity patterns
- Connect atomic structure to real-world chemistry
Whether you’re learning chemistry for exams or applying it in advanced scientific fields, mastering this trend builds a strong conceptual foundation that will serve you throughout your studies and beyond.
Finally, we suggest checking out The Reca Blog for more insightful blogs.